Abstract
Introduction: Daratumumab is an anti-CD38 monoclonal antibody, currently FDA approved for treatment of newly diagnosed transplant ineligible multiple myeloma (MM) in combination with melphalan, prednisone and bortezomib, in combination with lenalidomide-dexamethasone and bortezomib-dexamethasone for early relapse MM, and in combination with pomalidomide-dexamethasone and monotherapy for relapsed/refractory MM (RRMM). Recent data demonstrated the feasibility of infusing daratumumab at an accelerated rate over 90 minutes from Cycle1 day 15 onwards [1], reducing the standard infusion time of 3-4 hours. Considering these data, all daratumumab protocols at our institution were updated to implement the rapid infusion protocol in routine clinical practice. Herein, we report the safety profile of rapid daratumumab infusion at our center.
Methods: Retrospective chart review was performed from April 2016 through July 2018 to identify patients who completed at least one cycle of daratumumab or any daratumumab containing regimen for RRMM or amyloidosis at the Levine Cancer Institute (LCI). LCI includes a large infusion center at the main tertiary center and several other small infusion centers distributed throughout the states of North and South Carolina. Patients were divided into two cohorts; cohort 1 included patients who received daratumumab administered at the standard infusion rate recommended by manufacturer, whereas cohort 2 received rapid infusion daratumumab following the institution-wide implementation of the rapid infusion protocol in March 2018. Cohort 2 patients started on daratumumab received the first two doses of cycle 1 at the standard infusion rate followed by a 90-minute infusion with third and subsequent doses. Furthermore, 20% of dose was given over 30 minutes and the remaining 80% over 60 minutes (overall duration: 90 minutes). Patients in both cohorts were pre-medicated with: acetaminophen, diphenhydramine, dexamethasone and montelukast (for first two doses of first cycle), given 30 minutes prior to initiating daratumumab. The primary endpoint was to compare rates of infusion related reactions between the rapid and standard daratumumab infusion protocols using Fischer's exact test. Data collected included but not limited to: patient demographics, comorbidities, plasma cell disorder, pre-medications, type and management of infusion reactions, as well as post-infusion monitoring if documented.
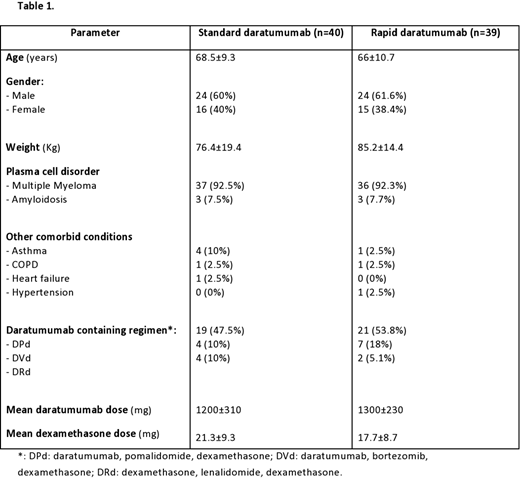
Results: A total of 73 RRMM (37 in cohort 1 and 36 in cohort 2) and 6 amyloidosis patients (3 in each cohort) were included in this study. Baseline characteristics are shown in table 1. Overall, there was no statistically significant difference in the rates of infusion related reactions between the rapid and standard infusion cohorts (2.6% vs. 5.0%, p=0.6). Of 39 patients in cohort 2, 8 patients received rapid infusion daratumumab on day 15 (3rd dose) of first cycle, whereas remaining received a median of 7 cycles (range: 2-30) before the switch was commenced. Among those 8 patients where daratumumab was started on day 15, only 1 developed an infusion related reaction. Notably, this patient had a history of prior exposure, and subsequently daratumumab was administered at the rapid infusion rate on day 1 instead of day 15, approximately 4 months later. All infusion reactions reported in both cohorts were grade 1 which were managed according to institutional guidelines followed by resumption of infusion at lower rates.
Conclusion and future directions: Our findings indicate that rapid daratumumab infusion beyond day 8 of cycle 1 is a safe, tolerable and convenient option for RRMM and amyloidosis patients. Additional studies are underway to evaluate cost benefits of rapid infusion daratumumab as well as patient/provider satisfaction.
References:
Barr H, Dempsey J, Waller A, et al. Ninety-minute Daratumumab Infusion is Safe in Multiple Myeloma. Leukemia. March 2018. DOI:10.1038/s41375-018-0120-2
Voorhees:Novartis: Consultancy, Other: served on an IRC; Oncopeptides: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: served on an IRC; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: served on an IRC; TeneoBio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen Inc.: Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees. Usmani:Abbvie, Amgen, Celgene, Genmab, Merck, MundiPharma, Janssen, Seattle Genetics: Consultancy; Amgen, BMS, Celgene, Janssen, Merck, Pharmacyclics,Sanofi, Seattle Genetics, Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal